As per IATF and QMS standard, there are a requirement of document and record control in our organization. Therefore, master list of documents and records plays important role to maintain organizational QMS system adherence and compliance.
When auditor will ask the requirement of documents control then you should present such list to auditor.
Therefore, this article we will learn everything about the master list of documents and record.
You can later download the free template or report of master list format.
Let’s dive into the topic.

Purpose | Why we create document master list? #
First point is due to the Quality management system documentation requirement. And why it is a requirement?
Because in organization there are many documents, records, and formats available. If you don’t have control over those all documents, then there are chances of not following correct documents and system withing company.
Possibility is that everyone can use their own method to record the evidence and no control on documentation, which is wrong.
This again leads to miss some important information or process causing non fulfilling customer requirements.
Therefore, it is important to have a organized master list of documents with their proper version control.

How to create master list of documents? #
The name itself explanatory that this is the list of all documents and records. The creation of master list of documents includes many stages such as,
- Each document or record creation
- Review and approval
- Communication or distribution
- Revision control
- Archiving or obsolete control
First we create many documents based on need within the organization.
Then the document is been release based on reviews and approvals by process owners.
These documents or records then added into the master list of documents and then communicated to the respective place for use to the users.
The list helps to maintain revisions and obsolete copies control if new version are release.
What are the contents of master list of documents and records? #
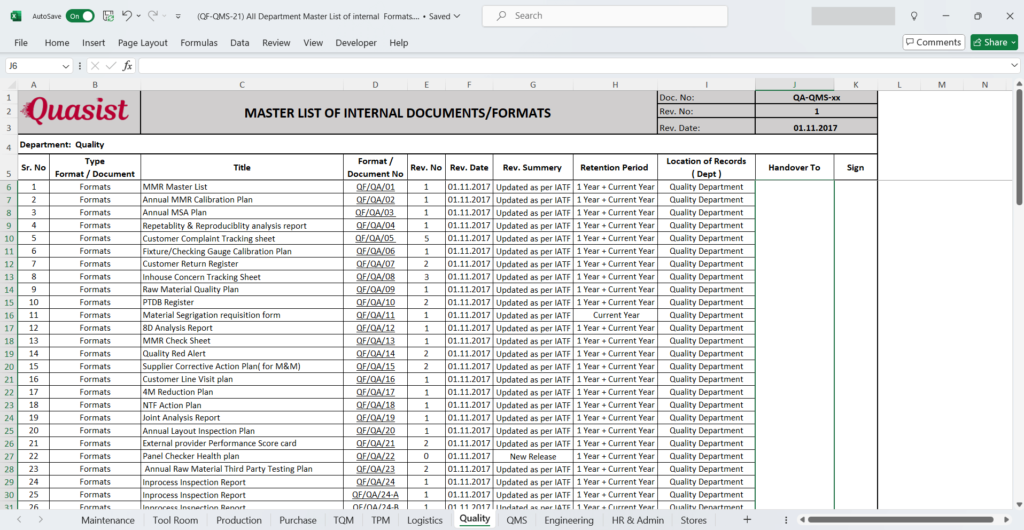
There are many columns in master list,
Type (Document / Record or Format): Mention correct type, such as document or record.
Title: Mention title of document or record.
Document/Format Number: Each document or format must have a unique identification number. So, please mention correct document/format number.
Revision Number: Along with number revision history also required to track the traceability of updates. Therefore it is important to mention revision number. Goal is everyone should follow correct revision of document or record.
Revision Date: All revisions come along with revision date. Mention same date of revision in respective column.
Revision Summary: Shortly you can explain what the reason of new revision is. Or just mention the change update.
Retention Period: This is something important from QMS standard perspective as every document and record have a retention period as per record retention policy. Mention the specific retention period required for respective document/record.
Location: Which location in organization this document/format used? Wither it can be the department or process location or on specific machine or area.
Handover To / Owner: The person who owns this documents and have responsibility to use correct document/record within their authority. Person can be process owner or Quality engineer, etc.
Which documents need add in master list? #
With all above fields you need to mention all your QMS documentation with specific category add it to this master list. Which includes,
- Quality Policy
- Quality Manual
- Procedures (Mandatory or non-mandatory)
- Work Instructions
- Documents and Records
Conclusion #
Best question from every QMS auditor while reviewing the QMS system is show me the master list of documents or records. Therefore its important to maintain your documents and records using this master list. This also maintain you QMS documentation flow according to standard and customer requirement.




